Red Blood Cells Harnessed as Nanoparticle Carriers for Vaccines
j July 20, 2020
July 14, 2020
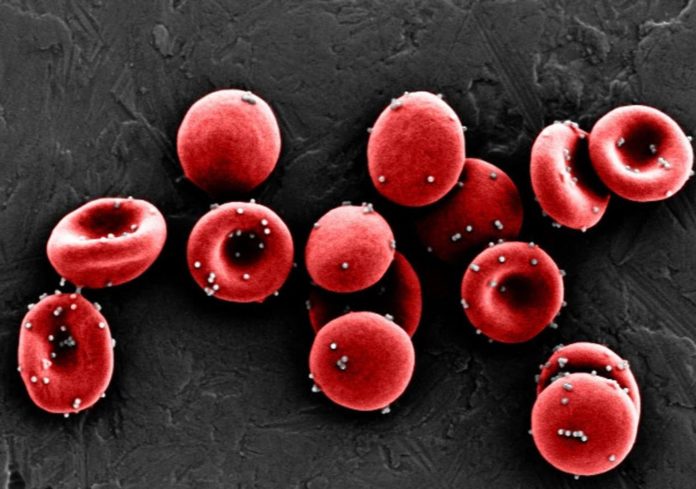
Source: Wyss Institute at Harvard University
Researchers led by a team at Harvard’s Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS) have harnessed an innate immune function of red blood cells to build a platform technology that uses erythrocytes to deliver antigens to immune system antigen-presenting cells (APCs) in the spleen, generating an immune response. Initial experiments showed that the technology, which they’ve called erythrocyte-driven immune targeting (EDIT), successfully slowed the growth of cancerous tumors in mice. The team suggests that the nanoparticle-carrying red blood cells could be used as a biocompatible adjuvant for a variety of vaccines.
“The spleen is one of the best organs in the body to target when generating an immune response because it is one of the few organs where red and white blood cells naturally interact,” said senior author Samir Mitragotri, PhD, a Wyss core faculty member who is also the Hiller professor of bioengineering and Hansjörg Wyss professor of biologically inspired engineering at SEAS. “Red blood cells’ innate ability to transfer attached pathogens to immune cells has only recently been discovered, and this study unlocks the door to an exciting array of future developments in the field of using human cells for disease treatment and prevention.” Mitragori’s team, and colleagues at the Perelman School of Medicine University of Pennsylvania, reported on their technology in the Proceedings of the National Academy of Sciences (PNAS), in a paper titled, “Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function.”
Red blood cells account for more than 80% of cells in the human body, and their primary role is to shuttle oxygen from our lungs to our organs, the authors wrote. However, erythrocytes are also “an active member of the innate immune system,” and can help the body to fight off infections. “Erythrocytes naturally capture certain bacterial pathogens in circulation, kill them through oxidative stress, and present them to the APCs in the spleen,” the team explained. “This offers a genuine opportunity to develop a biomimetic strategy to target spleen, erythrocyte-driven immune targeting, which leverages antigen presentation to the spleen from the surface of the erythrocyte.”
In fact, using red blood cells as delivery vehicles for drugs is not a new idea, but the vast majority of existing technologies target the lungs, because their dense network of capillaries causes the cargoes to shear off of red blood cells as they squeeze through the tiny vessels. “This makes it challenging to deliver the cargo to the spleen,” the investigators continued. “Reducing lung uptake is essential to enabling nanoparticle-carrying erythrocytes to escape the lungs and deliver their cargo to other organs, in this case, spleen.”
So, to make their strategy work Mitragotri’s research team first needed to figure out how to get antigens to stick to red blood cells strongly enough to resist shearing off in the lungs, so that they could reach the spleen. They coated polystyrene nanoparticles with ovalbumin, an antigenic protein known to cause a mild immune response, then incubated them with mouse red blood cells. They found that a ratio of 300 nanoparticles per blood cell resulted in the greatest number of nanoparticles bound to the cells, retention of about 80% of the nanoparticles when the cells were exposed to the shear stress found in lung capillaries, and moderate expression of a lipid molecule called phosphatidyl serine (PS) on the cells’ membranes.
“A high level of PS on red blood cells is essentially an ‘eat me’ signal that causes them to be digested by the spleen when they are stressed or damaged, which we wanted to avoid,” said Anvay Ukidve, a graduate student in the Mitragotri lab and co-first author of the paper. “We hoped that a lower amount of PS would instead temporarily signal ‘check me out’ to the spleen’s APCs, which would then take up the red blood cells’ antigen-coated nanoparticles without the cells themselves getting destroyed.”
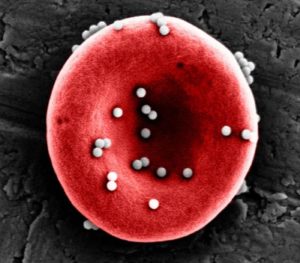
Nanoparticles coated in an antigen stick to red blood cells strongly enough to resist being sheared off in the lungs, allowing them to reach the spleen and be passed off to immune cells, initiating an antigen-specific immune response. Credit: Wyss Institute at Harvard University
via Red Blood Cells Harnessed as Nanoparticle Carriers for Vaccines
To test that hypothesis, the team injected red blood cells coated with their nanoparticles into mice, then tracked where they accumulated in their bodies. Twenty minutes after injection, more than 99% of the nanoparticles had been cleared from the animals’ blood, and more nanoparticles were present in their spleens than their lungs. The higher nanoparticle accumulation in the spleen persisted for up to 24 hours and the number of EDIT red blood cells in the circulation remained unchanged, showing that the red blood cells had successfully delivered their cargoes to the spleen without being destroyed
Having confirmed that their nanoparticles could be successfully delivered to the spleen in vivo, the researchers next evaluated whether the antigens on the nanoparticles’ surfaces induced an immune response. Mice were injected with EDIT once a week for three weeks, and then their spleen cells were analyzed. Treated mice displayed 8-fold and 2.2-fold more T cells displaying the delivered ovalbumin antigen than mice that were given “free” nanoparticles or were untreated, respectively. Mice treated with EDIT also produced more antibodies against ovalbumin in their blood than either of the other groups of mice.
To see if these EDIT-induced immune responses could potentially prevent or treat disease, the team repeated their three-week prophylactic injection of EDIT into mice, then inoculated them with lymphoma cells that expressed ovalbumin on their surfaces. The mice that received EDIT had about 3-fold slower tumor growth compared with the control group, and the group that received free nanoparticles, and had lower numbers of viable cancerous cells. This outcome significantly increased the window of time during which the tumor could be treated before the mice developed the disease. “Remarkably,” the scientists continued, “one mouse from the EDIT group remained tumor-free throughout the course of the study … EDIT significantly prolonged the tumor exponentiation, thereby increasing the window for therapeutic interventions with alternate strategies.”
“EDIT essentially is an adjuvant-free vaccine platform,” said Zongmin Zhao, PhD, a postdoctoral fellow in the Mitragotri lab and co-first author of the paper. “Part of the reason why vaccine development today takes so long is that foreign adjuvants delivered along with an antigen have to go through a full clinical safety trial for each new vaccine. “Red blood cells have been safely transfused into patients for centuries, and their ability to enhance immune responses could make them a safe alternative to foreign adjuvants, increasing the efficacy of vaccines and speed of vaccine creation.”
The team is continuing to work on understanding exactly how an immune response that is specific to the antigen presented by EDIT is generated by the spleen’s APCs, and plans to test it with other antigens beyond ovalbumin. They hope to use this additional insight to drive their pursuit of the optimal clinical setting(s) for the technology. “In summary, we have developed a biomimetic strategy that exploits the innate immune function of erythrocytes to engineer an efficient nanoparticle handoff to the spleen,” the authors concluded. “Fundamentally, it represents a pathway to deliver nanoparticles to the spleen that does not involve extensive modifications to the nanoparticles themselves … With further research and performing more specific immunological studies, this platform can be used as a versatile strategy to target several off-the-shelf nanoparticles to the spleen without specific modifications.”
“The human body is a treasure trove of elegant solutions to healthcare problems, and while medicine has come a long way in understanding those mechanisms, we are still in the early stages of being able to harness them to improve the length and quality of human life,” commented the Wyss Institute’s founding director Donald Ingber, MD, PhD, who is also the Judah Folkman professor of vascular biology at Harvard Medical School and Boston Children’s Hospital, and professor of bioengineering at SEAS. This research is an exciting step forward toward that goal, and could dramatically change how immune responses are modulated in patients.”
The authors further wrote, “Adjuvant-free therapies based on the ‘self’ cell of the body represent a unique way of propelling development of vaccines. While future studies can focus on understanding the similarities and differences between EDIT and other adjuvants, availability of additional adjuvants, especially self-based ones like perturbed erythrocytes, may significantly benefit the scientific community engaged in adjuvant and vaccine research.”
via Red Blood Cells Harnessed as Nanoparticle Carriers for Vaccines
https://jonsnewplace.wordpress.com/2020/07/20/red-blood-cells-harnessed-as-nanoparticle-carriers-for-vaccines/
Thanks to: https://jonsnewplace.wordpress.com
j July 20, 2020
July 14, 2020

Source: Wyss Institute at Harvard University
Researchers led by a team at Harvard’s Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS) have harnessed an innate immune function of red blood cells to build a platform technology that uses erythrocytes to deliver antigens to immune system antigen-presenting cells (APCs) in the spleen, generating an immune response. Initial experiments showed that the technology, which they’ve called erythrocyte-driven immune targeting (EDIT), successfully slowed the growth of cancerous tumors in mice. The team suggests that the nanoparticle-carrying red blood cells could be used as a biocompatible adjuvant for a variety of vaccines.
“The spleen is one of the best organs in the body to target when generating an immune response because it is one of the few organs where red and white blood cells naturally interact,” said senior author Samir Mitragotri, PhD, a Wyss core faculty member who is also the Hiller professor of bioengineering and Hansjörg Wyss professor of biologically inspired engineering at SEAS. “Red blood cells’ innate ability to transfer attached pathogens to immune cells has only recently been discovered, and this study unlocks the door to an exciting array of future developments in the field of using human cells for disease treatment and prevention.” Mitragori’s team, and colleagues at the Perelman School of Medicine University of Pennsylvania, reported on their technology in the Proceedings of the National Academy of Sciences (PNAS), in a paper titled, “Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function.”
Red blood cells account for more than 80% of cells in the human body, and their primary role is to shuttle oxygen from our lungs to our organs, the authors wrote. However, erythrocytes are also “an active member of the innate immune system,” and can help the body to fight off infections. “Erythrocytes naturally capture certain bacterial pathogens in circulation, kill them through oxidative stress, and present them to the APCs in the spleen,” the team explained. “This offers a genuine opportunity to develop a biomimetic strategy to target spleen, erythrocyte-driven immune targeting, which leverages antigen presentation to the spleen from the surface of the erythrocyte.”
In fact, using red blood cells as delivery vehicles for drugs is not a new idea, but the vast majority of existing technologies target the lungs, because their dense network of capillaries causes the cargoes to shear off of red blood cells as they squeeze through the tiny vessels. “This makes it challenging to deliver the cargo to the spleen,” the investigators continued. “Reducing lung uptake is essential to enabling nanoparticle-carrying erythrocytes to escape the lungs and deliver their cargo to other organs, in this case, spleen.”
So, to make their strategy work Mitragotri’s research team first needed to figure out how to get antigens to stick to red blood cells strongly enough to resist shearing off in the lungs, so that they could reach the spleen. They coated polystyrene nanoparticles with ovalbumin, an antigenic protein known to cause a mild immune response, then incubated them with mouse red blood cells. They found that a ratio of 300 nanoparticles per blood cell resulted in the greatest number of nanoparticles bound to the cells, retention of about 80% of the nanoparticles when the cells were exposed to the shear stress found in lung capillaries, and moderate expression of a lipid molecule called phosphatidyl serine (PS) on the cells’ membranes.
“A high level of PS on red blood cells is essentially an ‘eat me’ signal that causes them to be digested by the spleen when they are stressed or damaged, which we wanted to avoid,” said Anvay Ukidve, a graduate student in the Mitragotri lab and co-first author of the paper. “We hoped that a lower amount of PS would instead temporarily signal ‘check me out’ to the spleen’s APCs, which would then take up the red blood cells’ antigen-coated nanoparticles without the cells themselves getting destroyed.”

Nanoparticles coated in an antigen stick to red blood cells strongly enough to resist being sheared off in the lungs, allowing them to reach the spleen and be passed off to immune cells, initiating an antigen-specific immune response. Credit: Wyss Institute at Harvard University
via Red Blood Cells Harnessed as Nanoparticle Carriers for Vaccines
To test that hypothesis, the team injected red blood cells coated with their nanoparticles into mice, then tracked where they accumulated in their bodies. Twenty minutes after injection, more than 99% of the nanoparticles had been cleared from the animals’ blood, and more nanoparticles were present in their spleens than their lungs. The higher nanoparticle accumulation in the spleen persisted for up to 24 hours and the number of EDIT red blood cells in the circulation remained unchanged, showing that the red blood cells had successfully delivered their cargoes to the spleen without being destroyed
Having confirmed that their nanoparticles could be successfully delivered to the spleen in vivo, the researchers next evaluated whether the antigens on the nanoparticles’ surfaces induced an immune response. Mice were injected with EDIT once a week for three weeks, and then their spleen cells were analyzed. Treated mice displayed 8-fold and 2.2-fold more T cells displaying the delivered ovalbumin antigen than mice that were given “free” nanoparticles or were untreated, respectively. Mice treated with EDIT also produced more antibodies against ovalbumin in their blood than either of the other groups of mice.
To see if these EDIT-induced immune responses could potentially prevent or treat disease, the team repeated their three-week prophylactic injection of EDIT into mice, then inoculated them with lymphoma cells that expressed ovalbumin on their surfaces. The mice that received EDIT had about 3-fold slower tumor growth compared with the control group, and the group that received free nanoparticles, and had lower numbers of viable cancerous cells. This outcome significantly increased the window of time during which the tumor could be treated before the mice developed the disease. “Remarkably,” the scientists continued, “one mouse from the EDIT group remained tumor-free throughout the course of the study … EDIT significantly prolonged the tumor exponentiation, thereby increasing the window for therapeutic interventions with alternate strategies.”
“EDIT essentially is an adjuvant-free vaccine platform,” said Zongmin Zhao, PhD, a postdoctoral fellow in the Mitragotri lab and co-first author of the paper. “Part of the reason why vaccine development today takes so long is that foreign adjuvants delivered along with an antigen have to go through a full clinical safety trial for each new vaccine. “Red blood cells have been safely transfused into patients for centuries, and their ability to enhance immune responses could make them a safe alternative to foreign adjuvants, increasing the efficacy of vaccines and speed of vaccine creation.”
The team is continuing to work on understanding exactly how an immune response that is specific to the antigen presented by EDIT is generated by the spleen’s APCs, and plans to test it with other antigens beyond ovalbumin. They hope to use this additional insight to drive their pursuit of the optimal clinical setting(s) for the technology. “In summary, we have developed a biomimetic strategy that exploits the innate immune function of erythrocytes to engineer an efficient nanoparticle handoff to the spleen,” the authors concluded. “Fundamentally, it represents a pathway to deliver nanoparticles to the spleen that does not involve extensive modifications to the nanoparticles themselves … With further research and performing more specific immunological studies, this platform can be used as a versatile strategy to target several off-the-shelf nanoparticles to the spleen without specific modifications.”
“The human body is a treasure trove of elegant solutions to healthcare problems, and while medicine has come a long way in understanding those mechanisms, we are still in the early stages of being able to harness them to improve the length and quality of human life,” commented the Wyss Institute’s founding director Donald Ingber, MD, PhD, who is also the Judah Folkman professor of vascular biology at Harvard Medical School and Boston Children’s Hospital, and professor of bioengineering at SEAS. This research is an exciting step forward toward that goal, and could dramatically change how immune responses are modulated in patients.”
The authors further wrote, “Adjuvant-free therapies based on the ‘self’ cell of the body represent a unique way of propelling development of vaccines. While future studies can focus on understanding the similarities and differences between EDIT and other adjuvants, availability of additional adjuvants, especially self-based ones like perturbed erythrocytes, may significantly benefit the scientific community engaged in adjuvant and vaccine research.”
via Red Blood Cells Harnessed as Nanoparticle Carriers for Vaccines
https://jonsnewplace.wordpress.com/2020/07/20/red-blood-cells-harnessed-as-nanoparticle-carriers-for-vaccines/
Thanks to: https://jonsnewplace.wordpress.com






 Sat Mar 23, 2024 11:33 pm by globalturbo
Sat Mar 23, 2024 11:33 pm by globalturbo

