Johnson & Johnson Latest To Halt COVID-19 Vaccine Trial Over Unspecified Illness
Posted on October 12,
By Tyler Durden
Yet another high-profile Phase 3 vaccine trial has been temporarily halted after one of the participants developed a suspicious illness.
According to a report published Monday night by STAT News, Johnson & Johnson has informed participants and researchers that its 60,000-person trial would be temporarily paused as the company and the Data and Safety Monitoring Board, the organization overseeing all the US COVID-19 trials.
JNJ confirmed the pause when contacted by STAT, though it offered no details about the illness or the patient.
Contacted by STAT, J&J confirmed the study pause, saying it was due to “an unexplained illness in a study participant.”
Pauses like these aren’t uncommon in vaccine trials.
“If we do a study of 60,000 people, that is a small village,” the source said. “In a small village there are a lot of medical events that happen.”
But these trials are drawing more scrutiny ever since the AstraZeneca-Oxford trial was put on hold by regulators in the UK after a participant was sickened with symptoms of what was believed to be transverse myelitis, a serious spinal issue. Trials resumed in the UK, India and elsewhere days later, but in the US, an AZ trial remains on hold due to an unspecified issue. Both AZ and US regulators have been suspiciously tight-lipped.
Already, public health officials in the US, Europe and around the world are worried about waning confidence in the vaccine, with some surveys showing that roughly half the public would rather not take it.
In a research note published earlier, analysts at Goldman Sachs wrote that trust in the vaccine could be a serious barrier to its ultimate eradication.
Futures ticked lower on the news.
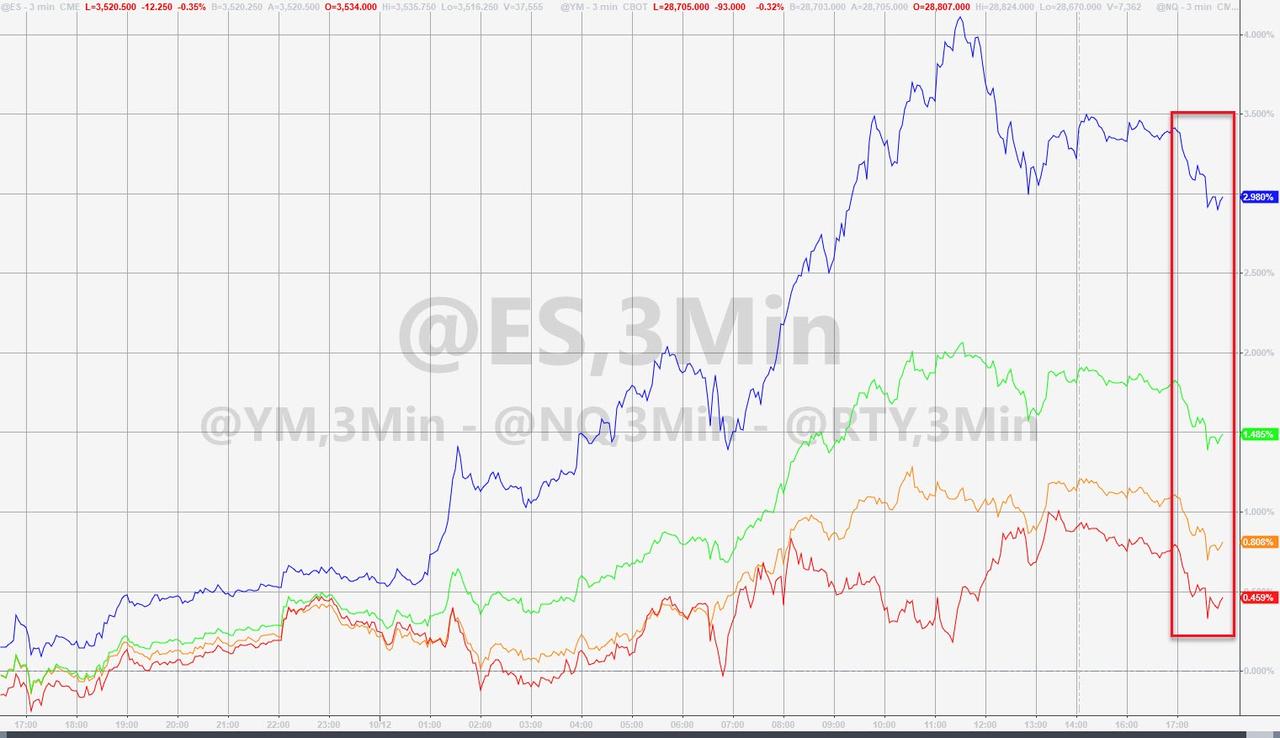
But there was no evidence of the intense selling pressure that followed news about the initial AstraZeneca-Oxford vaccine project halt.
Source: Zerohedge
Image: The Last American Vagabond
https://www.naturalblaze.com/2020/10/johnson-johnson-latest-to-halt-covid-19-vaccine-trial-over-unspecified-illness.html?utm_source=Natural+Blaze+Subscribers&utm_medium=email&utm_campaign=72e0a43da2-RSS_EMAIL_CAMPAIGN&utm_term=0_b73c66b129-72e0a43da2-388109773
Thanks to: https://www.naturalblaze.com
Posted on October 12,

By Tyler Durden
Yet another high-profile Phase 3 vaccine trial has been temporarily halted after one of the participants developed a suspicious illness.
According to a report published Monday night by STAT News, Johnson & Johnson has informed participants and researchers that its 60,000-person trial would be temporarily paused as the company and the Data and Safety Monitoring Board, the organization overseeing all the US COVID-19 trials.
JNJ confirmed the pause when contacted by STAT, though it offered no details about the illness or the patient.
Contacted by STAT, J&J confirmed the study pause, saying it was due to “an unexplained illness in a study participant.”
According to STAT, the DSMB was convened late Monday evening to start looking into the case. J&J said that in cases like this, “it is not always immediately apparent” whether the participant who experienced an adverse event received the experimental vaccine, or a placebo.The company declined to provide further details. “We must respect this participant’s privacy. We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information,” the company said in a statement.
Pauses like these aren’t uncommon in vaccine trials.
“If we do a study of 60,000 people, that is a small village,” the source said. “In a small village there are a lot of medical events that happen.”
But these trials are drawing more scrutiny ever since the AstraZeneca-Oxford trial was put on hold by regulators in the UK after a participant was sickened with symptoms of what was believed to be transverse myelitis, a serious spinal issue. Trials resumed in the UK, India and elsewhere days later, but in the US, an AZ trial remains on hold due to an unspecified issue. Both AZ and US regulators have been suspiciously tight-lipped.
Already, public health officials in the US, Europe and around the world are worried about waning confidence in the vaccine, with some surveys showing that roughly half the public would rather not take it.
In a research note published earlier, analysts at Goldman Sachs wrote that trust in the vaccine could be a serious barrier to its ultimate eradication.
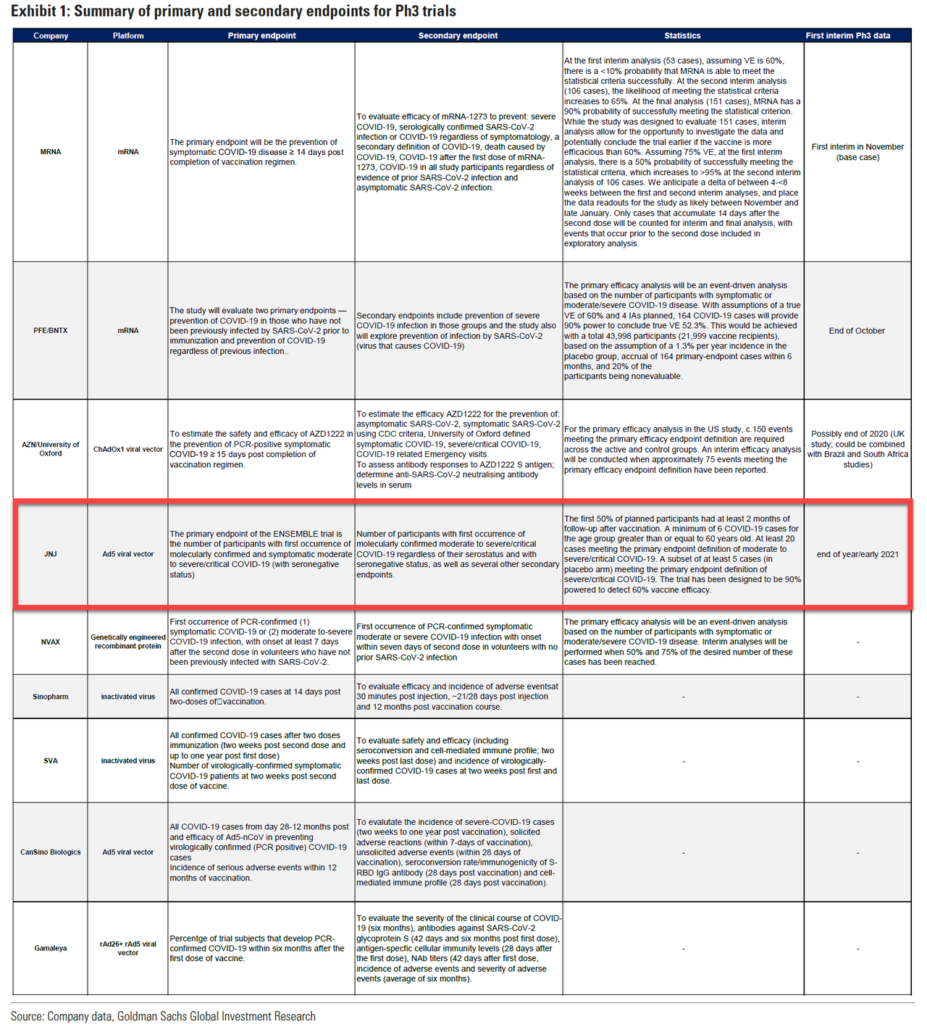
JNJ is using an adenovirus vector, like several other top vaccine projects.We think that the biggest challenge to ultimately lowering the disease burden and virus circulation to very low levels will be convincing the broad population to take the vaccine. Our base case assumes such broad uptake but this will likely require a safe and very effective vaccine, trust in the approval and rollout process, no out-of -pocket costs, and effective public and community campaigns.

Futures ticked lower on the news.

But there was no evidence of the intense selling pressure that followed news about the initial AstraZeneca-Oxford vaccine project halt.
Source: Zerohedge
Image: The Last American Vagabond
FREE PDF: 10 Best Books To Survive Food Shortages & Famines
https://www.naturalblaze.com/2020/10/johnson-johnson-latest-to-halt-covid-19-vaccine-trial-over-unspecified-illness.html?utm_source=Natural+Blaze+Subscribers&utm_medium=email&utm_campaign=72e0a43da2-RSS_EMAIL_CAMPAIGN&utm_term=0_b73c66b129-72e0a43da2-388109773
Thanks to: https://www.naturalblaze.com






 Sat Mar 23, 2024 11:33 pm by globalturbo
Sat Mar 23, 2024 11:33 pm by globalturbo

