
Beware the Snake Oil Salesmen
“The snake venom theory by Dr. Bryan Ardis is built upon the interpretation of the unpurified fraudulent
“SARS-COV-2” genome which is itself built upon references to other fraudulent genomes of human and
animal “coronaviruses” created in the very same way. Attempting to claim any connections between the
random A,C,T,G’s in a computer database is a useless and pointless exercise as the RNA that was fabricated
into the genome of a “virus” was never purified, isolated, and proven to physically exist in the first place.
Thus any connections between the protein codes said to belong to a “virus” which are then said to be closely
related to supposed snake “coronaviruses” is immediately invalid.
Using this invalid premise to then claim that people have been poisoned by snake venom in the vaccines,
the drugs, and the water supply is nothing but unsubstantiated science fiction that seems designed to have
a few purposes:
- To keep people engaged in the lie that a new disease known as “Covid-19” exists and that there is a
singular cause. - To restore faith in monoclonal antibodies and other toxic alternative treatments.
- To use the theory to promote and sell anti-venom supplements.
- To divide and distract those questioning the official narrative.
- To make the “Truther” community look foolish by falling for loosely tied-together circumstantial
evidence that is easily debunked.”
~ Mike Stone
Beware the Snake Oil Salesmen
by Mike Stone, Viroliegy
April 18, 2022
“My story has never been to create fear, panic, and anxiety about water.” He said he told Peters that he believes “there’s actually a snake venom connection to all of COVID-19, and I think that’s the weapon.” – Dr. Bryan Ardis
https://www.thedailybeast.com
Summarizing his theory, Dr. Ardis said, “They are using Krait venom and Cobra venom, calling it Covid-19, you’re drinking it, it’s getting into your brainstem and it’s paralyzing your diaphragm’s ability to breathe.”
https://www.google.com/amp/s/miamistandard.news/
I really didn’t want to write this article. I was hopeful that people would easily see right through the unsubstantiated claims of Dr. Bryan Ardis that snake venom is the cause of “Covid.” I was hopeful that people would take the time to research the information presented in support of the snake venom theory to see if it held any merit at all. I thought his whirlwind alternative media tour on the who’s who of questionable sources (including the likes of Stew Peters, Mike Adams, and Infowars) would have people questioning why this theory was allowed to be so heavily promoted so quickly. I thought that the fact that the man who created the “Covid” snake venom theory was actually selling his own anti-venom line of supplements would be enough grounds to be skeptical of his motive and his claims.
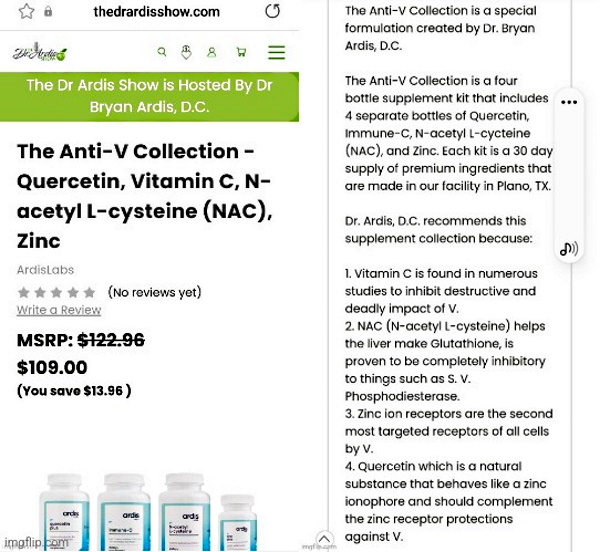
It seems I was wrong. Just like the baseless vaccine shedding and gain of function/bioweapons narratives, this new snake venom theory has sadly spread through the “Truther” community like wildfire, with many who rightfully challenge the existence of “viruses” clinging to the idea of a new invisible enemy to defeat. They believe that it must be a new toxin. It can’t possibly be the same factors we have seen each and every year leading to disease. This toxin must be hiding in the vaccines, the drugs, and/or even the very water we drink. What these “Truthers” do not realize is that this very line of thinking gives credibility to the idea of a new disease which requires new treatments in order to combat it. This is exactly what the pharmaceutical companies want you to believe.
However, there is NO NEW DISEASE. There is no need for any new or even existing pharmaceutical interventions to treat the same symptoms of detoxification people go through each and every year. In fact, the current treatments can easily be shown to have led to numerous unnecessary deaths. There is no new threat known as “Covid-19” which is being caused by any one factor. The factors leading to the symptoms of disease people are experiencing are multi-causal as they are every year.
Now this is not to say that the vaccines, the drugs, or even the water supply are free of toxins. These are all sources of toxicity and should be investigated as to their composition and effects on our health. However, the theory that there is one factor in all of these sources, i.e. snake venom, and this one factor is leading to the symptoms of disease people are experiencing is, at present time, completely baseless. And it all begins at the very foundation of the fraudulent genome.
The Fradulent Genome
You take that snake or that serpent and you figure out how to isolate genes from that serpent and get those genes of that serpent to insert itself into your God-given created DNA. I think this is the plan all along, was to get the serpents’, the evil one’s DNA, into your God-created DNA.”
https://www.thedailybeast.com/covid-conspiracy-theorists-are-at-war-over-snake-venom
He also said genetic sequence testing done on sick patients in Wuhan found their genetic sequence matched two snakes, the Chinese Krait and King Cobra, not bats.”
https://www.google.com/amp/s/miamistandard.news/
From Dr. Ardis’ interview with Mike Adams, he supplied the article “Snakes could be the source of the Wuhan coronavirus outbreak” from CNN as his starting point for the “Covid”/snake connection. Within the article, you can see that this claim originates from the fraudulent genomes:
“The researchers used an analysis of the protein codes favored by the new coronavirus and compared it to the protein codes from coronaviruses found in different animal hosts, like birds, snakes, marmots, hedgehogs, manis, bats and humans. Surprisingly, they found that the protein codes in the 2019-nCoV are most similar to those used in snakes.”
https://www.google.com/amp/s/amp.cnn.com/
To anyone who actually researched the creation of the original “SARS-COV-2” genome, it is readily apparent that it is a fraudulent computer-generated creation stemming from the unpurified lung fluid of a single patient. The sequenced material could have come from multiple sources, including host DNA/RNA, bacteria, and microbes/microorganisms. It could have even come from outside contamination. There is no way to tell what the origin of the RNA is or even if it was a single source as no particles assumed to be “SARS-COV-2” were ever properly purified and isolated directly from the fluids of the sick patient before being sequenced. Thus, any relation this fabricated sequence has to any other sequence is invalid as the source was never identified to exist as a physical entity to begin with. Considering that the bat and snake “coronavirus” sequences for which the “SARS-COV-2” sequence was then compared to also come from unpurified sources, it is easy to see that any claims as to the origins of the sequenced material is a horrible foundation to build upon for an origin theory of a nonexistent “virus” and/or disease.
Even if this snake-venom connection was valid, the enzyme phospholipase A2 group IIA or sPLA2-IIA, which Dr. Ardis bases much of his claims on, only has similarities to rattlesnake venom. These peptides are “almost identical” to the venoms of animals and yet they are regularly found in healthy humans and other mammals. From his own source:
Like Venom Coursing Through the Body: Researchers Identify Mechanism Driving COVID-19 Mortality
“Researchers from the University of Arizona, in collaboration with Stony Brook University and Wake Forest School of Medicine, analyzed blood samples from two COVID-19 patient cohorts and found that circulation of the enzyme – secreted phospholipase A2 group IIA, or sPLA2-IIA, – may be the most important factor in predicting which patients with severe COVID-19 eventually succumb to the virus.
The sPLA2-IIA enzyme, which has similarities to an active enzyme in rattlesnake venom, is found in low concentrations in healthy individuals and has long been known to play a critical role in defense against bacterial infections, destroying microbial cell membranes.”
Thus, the snake enzymes are in fact normal human enzymes that are regularly found in healthy individuals. There is no mystery as to why these would be present in a sample. We should be able to put this “Covid” snake venom nonsense to bed right here. However, let’s press on a see what else we can uncover.
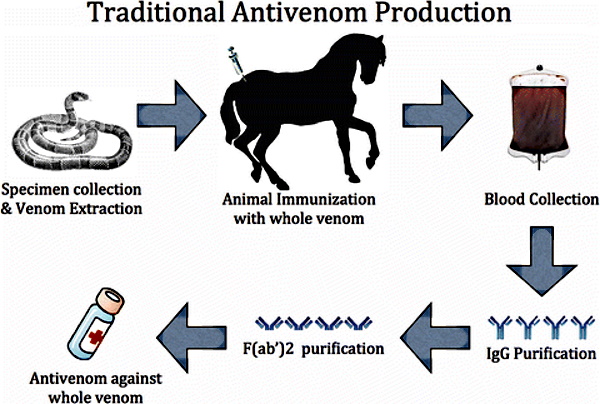
Antivenom = Monoclonal Antibodies
One thing I will give Dr. Ardis credit for is spotlighting the connection between the creation of antivenoms with the creation of monoclonal antibodies. The processes for both are very similar and the desired outcome is the exact same: the creation of theoretical antibodies. In the case of snake antivenom, it is normally created by a series of injections of the venom of a snake into an animal and then collecting the blood after a period of time. This is usually done through horses but other animals can be used as the host as well. Thus, the antivenom used for a snakebite victim is typically an injection of horse blood.
Monoclonal antibodies, on the other hand, are created by injecting mice with an antigen, extracting the resulting blood which contains the theoretical antibodies, and culturing it in myeloma (i.e. cancer) cells. For the creation of “SARS-COV-2” therapies, it is said that they are typically created either from the B cells of recovered “Covid” patients or by immunizing mice genetically modified to have a humanized immune system and harvesting the “effective” antibodies from them.
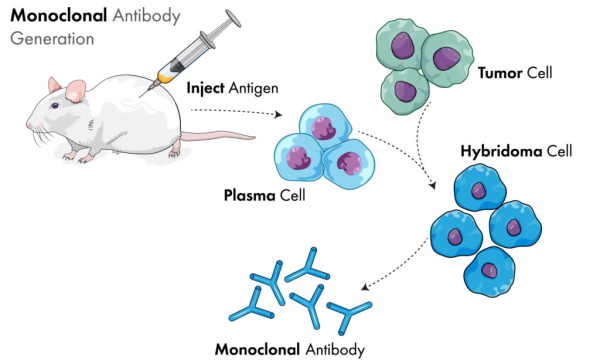
Both of these therapies have their basis in animal blood and the creation of the theoretical antibodies. Both are associated with toxic side effects. Sadly, while he was originally right about the fact that monoclonal antibodies are toxic and should not be used to treat the symptoms now collectively known as “Covid,” Dr. Ardis changed his tune when another doctor texted him asking if he would use antivenom for a snake bite:
“Last December, Dr Bryan Ardis received a text message from an Emergency Room physician friend of his that sent him down an unexpected and bizarre rabbit hole that may explain the adverse events from the vaccines that we’ve been reporting. The text read: “Hey Dr Ardis…If you got bit by a rattlesnake, would you go to a hospital and get anti-venom?”
“He says, “I realized, all of a sudden, monoclonal antibodies ARE anti-venom. The Federal Government doesn’t want us using anti-venom. Why are they fighting anti-venom and why are we finding anti-venom works against COVID? Is it not a virus? Is it a venom? This is what I want to know: Is COVID a venom and is this why they don’t want you using monoclonal antibodies?”
https://www.google.com/amp/s/cairnsnews.org/
Do you see the trick? They want you to equate monoclonal antibodies with antivenom. This is supposed to be an “aha” moment where you realize that there is no way that you would not inject antivenom (i.e. horse blood) into yourself if bitten by a snake. It’s a no-brainer, right? We have all seen the movies where a person is bitten by a venomous snake and quickly dies if not given the antivenom.
<span data-mce-type="bookmark" style="display: inline-block; width: 0px; overflow: hidden; line-height: 0;" class="mce_SELRES_start"></span>
If you are willing to accept the injection of horse blood into your body to survive a snake bite, why wouldn’t you also inject the cancer-cell cultured blood of genetically altered mice in order to combat “Covid?”
As Dr. Ardis points out, monoclonal antibodies are essentially antivenom. However, he wrongly states that monoclonal antibodies are an effective therapy. According to a September 2021 Cochrane review of the available studies, they found insufficient evidence to claim that monoclonal antibodies are an effective treatment for “SARS-COV-2:”
Are laboratory-made, COVID-19-specific monoclonal antibodies an effective treatment for COVID-19?
“The evidence for each comparison is based on single studies. None of these measured quality of life. Our certainty in the evidence for all non-hospitalised individuals is low, and for hospitalised individuals is very low to moderate. We consider the current evidence insufficient to draw meaningful conclusions regarding treatment with SARS-CoV-2-neutralising mAbs.”
https://www.cochrane.org/CD013825/
In other words, the evidence for the usefulness of monoclonal antibodies is non-existent. Unfortunately, the Cochrane Review failed to point out that there are various risks and adverse reactions associated with their use:
Do mAbs have risks?
“Therapeutic mAbs, typically administered by intravenous (IV) infusion, have been a valuable and generally safe treatment option for a variety of conditions for many years. However, they are also known to cause a range of side effects and reactions, which can be immediate or delayed. Serious adverse events associated with mAbs include infusion reactions, acute anaphylaxis, and serum sickness, as well as longer-term complications such as infections, cancer, autoimmune disease, and cardiotoxicity.”
https://www.ecri.org/components/
In January 2022, the FDA restricted the use of some monoclonal therapies (Bamlanivimab and Etesevimab) that are authorized against “Covid-19” as they were shown to be ineffective:
Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant
“In light of the most recent information and data available, today, the FDA revised the authorizations for two monoclonal antibody treatments – bamlanivimab and etesevimab (administered together) and REGEN-COV (casirivimab and imdevimab) – to limit their use to only when the patient is likely to have been infected with or exposed to a variant that is susceptible to these treatments.
Because data show these treatments are highly unlikely to be active against the omicron variant, which is circulating at a very high frequency throughout the United States, these treatments are not authorized for use in any U.S. states, territories, and jurisdictions at this time. In the future, if patients in certain geographic regions are likely to be infected or exposed to a variant that is susceptible to these treatments, then use of these treatments may be authorized in these regions.
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses, like SARS-CoV-2. And like other infectious organisms, SARS-CoV-2 can mutate over time, resulting in certain treatments not working against certain variants such as omicron. This is the case with these two treatments for which we’re making changes today.”
https://www.fda.gov/news-events/
On April 16th, 2022, the FDA revoked the use of Bamlanivimab alone as it’s benefits were shown not to outweigh its risks. Somehow despite this evidence, the FDA still allows for it to be used in combination with Etesevimab, even though they previously revoked their use together in January 2022:
Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab
“Today, the U.S. Food and Drug Administration revoked the emergency use authorization (EUA) that allowed for the investigational monoclonal antibody therapy bamlanivimab, when administered alone, to be used for the treatment of mild-to-moderate COVID-19 in adults and certain pediatric patients. Based on its ongoing analysis of emerging scientific data, specifically the sustained increase of SARS-CoV-2 viral variants that are resistant to bamlanivimab alone resulting in the increased risk for treatment failure, the FDA has determined that the known and potential benefits of bamlanivimab, when administered alone, no longer outweigh the known and potential risks for its authorized use. Therefore, the agency determined that the criteria for issuance of an authorization are no longer met and has revoked the EUA.
On Nov. 9, 2020, based on the totality of scientific evidence available at the time, the FDA issued an EUA to Eli Lilly and Co. authorizing the emergency use of bamlanivimab alone for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progressing to severe COVID-19 and/or hospitalization. Importantly, although the FDA is now revoking this EUA, alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab, administered together, for the same uses as previously authorized for bamlanivimab alone. The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19 when used in accordance with the authorized labeling based on information available at this time.”
https://www.fda.gov/news-events/press-announcements/
If the FDA’s confusing revoking of the EUA’s of these monoclonal antibodies has you concerned that you will not be able to use them against an imaginary “virus,” don’t worry. The FDA authorized the use of a new “Omicron-specific” monoclonal antibody called Bebtelovimab on February 11th, 2022. Granted, it still carries the same risks, adverse side effects, and uncertainty over clinical worsening listed for the previously ineffective antibody therapies. From the FDA fact sheet:
Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant
“Possible side effects of bebtelovimab include itching, rash, infusion-related reactions, nausea and vomiting. Serious and unexpected adverse events including hypersensitivity, anaphylaxis and infusion-related reactions have been observed with other SARS-CoV2 monoclonal antibodies and could occur with bebtelovimab. In addition, clinical worsening following administration of other SARS-CoV-2 monoclonal antibody treatment has been reported and therefore is possible with bebtelovimab. It is not known if these events were related to SARS-CoV-2 monoclonal antibody use or were due to progression of COVID-19.”
https://www.fda.gov/news-events/press-announcements/
Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant
- Hypersensitivity Including Anaphylaxis and Infusion-Related Reactions: Serious hypersensitivity reactions, including anaphylaxis, have been observed with administration of other SARS-CoV-2 monoclonal antibodies and could occur with administration of bebtelovimab. If clinically significant hypersensitivity reactions occur, discontinue and initiate appropriate supportive care. Infusion-related reactions may occur up to 24 hours post injection. These reactions may be severe or life threatening. (5.1)
- Clinical Worsening After SARS-CoV-2 Monoclonal Antibody Administration: Clinical worsening of COVID-19 after administration of SARS-CoV-2 monoclonal antibody treatment has been reported and may include signs or symptoms of fever, hypoxia or increased respiratory difficulty, arrhythmia (e.g., atrial fibrillation, sinus tachycardia, bradycardia), fatigue, and altered mental status. Some of these events required hospitalization. It is not known if these events were related to SARS-CoV-2 monoclonal antibody use or were due to progression of COVID-19. (5.2)
- Limitations of Benefit and Potential for Risk in Patients with Severe COVID-19: Treatment with bebtelovimab has not been studied in patients hospitalized due to COVID-19. Monoclonal antibodies, such as bebtelovimab, may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 requiring high flow oxygen or mechanical ventilation. (5.3)
http://www.fda.gov/media/156152/download
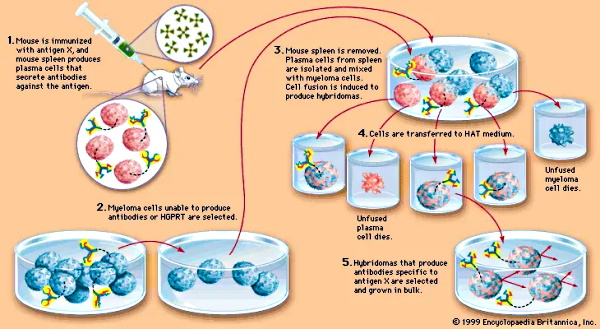 Mixed with cancer cells. Sounds healthy…
Mixed with cancer cells. Sounds healthy…It should be fairly clear that, unlike Dr. Ardis’ claims, monoclonal antibodies are not effective, carry numerous risky side effects, and can actually worsen the disease they are supposed to treat. Interestingly, this same risk of dangerous side effects and worsening disease outcomes is associated with snake antivenom as well. From the fact sheet of a commonly used antivenom for rattlesnake bites, we find these admitted side effects:
Rattlesnake Antivenin Side Effects Center
“Rattlesnake Antivenin (antivenin crotalidae polyvalent) is an antivenin product used only to treat envenomation caused by bites of crotalids (pit vipers) including rattlesnakes, copperhead and cottonmouth moccasins, and others. Common side effects of Rattlesnake Antivenin include allergic reactions such as flushing, itching, hives, swelling of the face/tongue/throat, cough, shortness of breath, blue color to the skin, vomiting, and anaphylaxis (severe allergic reaction).”
“Immediate systemic reactions (allergic reactions or anaphylaxis) can occur whenever a horse-serum-containing product is administered. An immediate reaction (e.g. shock, anaphylaxis) usually occurs within 30 minutes. Symptoms and signs may develop before the needle is withdrawn and may include apprehension, flushing, itching, urticaria; edema of the face, tongue, and throat; cough, dyspnea, cyanosis, vomiting, and collapse. There have been isolated reports of cardiac arrest and death associated with Antivenin (Crotalidae) Polyvalent (equine origin) use.”
“Serum sickness usually occurs 5 to 24 days after administration and its frequency may be related to the number of Antivenin vials administered.30 The incubation period may be less than 5 days, especially in those who have received horse-serum-containing preparations in the past. The usual symptoms and signs are malaise, fever, urticaria, lymphadenopathy, edema, arthralgia, nausea, and vomiting. Occasionally, neurological manifestations develop, such as meningismus or peripheral neuritis. Peripheral neuritis usually involves the shoulders and arms. Pain and muscle weakness are frequently present, and permanent atrophy may develop.”
https://www.rxlist.com/rattlesnake-antivenin-side-effects-drug-center.htm
Maybe the use of antivenom to treat a snakebite isn’t the super cure it has been sold to be? Is it possible that, as with many pharmaceutical products and interventions, the antivenom itself is creating the very symptoms it is said to treat? For some further insight, let’s look at a few highlights from an paper from September 2019, right before this “crisis,” which reviewed the use of antivenom and had a few revealing claims about the “anti” toxin. You will see it reiterated that the injection of antivenom created from either horse, sheep, goats, and/or rabbits can cause immediate hypersensitivity and anaphylaxis or a delayed “serum sickness” which can occur weeks after the treatment. It is stated that the antivenom has limited efficacy and can be entirely ineffective based on the geographic location. Improper use of antivenom contributes to increased servere outcomes and the production of antibodies in animals leads to a large number (70%) of immunoglobulins that do not react to snake venom:
Perspective on the Therapeutics of Anti-Snake Venom
3. Current Information in the Design of New Antivenoms
“Currently, the only accepted treatment for snakebite envenomation involves intravenous administration of conventional antivenoms comprising antibodies or antibody fragments derived from the plasma of large mammals (generally horses, but also sheep, goats, or rabbits) that have been previously immunized with non-lethal venomous doses [14,15]. Hyperimmunized animals produce antibodies against the venom proteins and serum is extracted from their blood for the treatment of envenomation [6,16]. Conventional serum therapy aims to bind and neutralize the snake venom proteins [17]. It is a fact that the antivenom allows the body to try to reverse the damage caused by the venom. However, it is known that such therapy can cause problems related to different antivenom characteristics, such as:
- Immediate hypersensitivity reaction to the alien immunoglobulins, including anaphylactic and pyrogenic reactions such as chills, rigor, headache, and tachycardia. Delayed antivenom reactions or serum sickness is observed after 8 to 12 days of treatment; these are characterized by cutaneous eruptions, fever, and allergies, among other effects [18];
- Limited efficacy of antivenom therapy to protect the affected organ/s against immediate local tissue damage and low stability;
- Ineffectiveness of the antivenom due to significant geographic variation in the composition of the venom;
- Antigenic reactivity due to the taxonomic diversity of the snakes;
- Improper use of the antivenom due to incorrect medical management, which contributes to a high incidence of adverse reactions, a low toxin neutralizing potency, or both.
“Current antibody production faces challenges during the immunization of the animal (equine or ovine), leading to the production of a huge number of antibodies that are not related to the snake venom. Around 70% of the immunoglobulins obtained do not act directly against venom toxins [26]. Despite the abovementioned facts, this is the only FDA approved therapy to treat snake venom.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6767026/
A few other studies also point out the severe reactions regularly attributed to the use of antivenom. The first is a study from 2016 which points out that not only are adverse reactions common, they occur at a high rate. It is stated that this is due to poor quality control and manufacturing problems:
Adverse reactions to snake antivenom, and their prevention and treatment
“Antivenom is the mainstay of treatment of snakebite envenoming. However, adverse reactions to snake antivenom that is available are common in many parts of the world where snakebite is prevalent. Both acute (anaphylactic or pyrogenic) and delayed (serum sickness type) reactions occur. Acute reactions are usually mild but severe systemic anaphylaxis may develop, often within an hour or so of exposure to antivenom. Serum sickness after antivenom has a delayed onset between 5 and 14 days after its administration. Ultimately, the prevention reactions will depend mainly on improving the quality of antivenom.”
“The high rate of acute adverse reactions to antivenom is an example of how poor manufacturing and quality control by antivenom producers cause problems for patients and their doctors. This highlights the importance of addressing issues related to poor quality and potentially unsafe antivenom. Ultimately, the prevention of reactions will depend mainly on improving the quality of antivenom. Until these improvements take place, doctors will have to depend on pharmacological prophylaxis as well as careful observation of patients receiving antivenom in preparation for prompt management of acute as well as delayed reactions when they occur.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4767202/
This next source is from 2018 and it points out that early antivenoms were unsafe and caused severe life-threatening events. While they now have “acceptable” safety profiles, antivenoms still have varying quality and range from 10% adverse reactions to greater than 50%. This same variation in quality is seen in the production of monoclonal antibodies:
Antivenom therapy: efficacy of premedication for the prevention of adverse reactions
“However, in their initial applications, antivenoms did not exhibit good safety results and could even cause life-threatening side effects [8]. The main reason was that first antivenoms were poorly purified preparations or crude sera. Over the years, for many of the original applications, heterologous serums were replaced by other drugs with better safety profiles, such as antibiotics, vaccines and homologous serums. However, in cases of envenomation by snakes, scorpions or arachnids, antivenoms remain the only effective treatment [4]. Currently, after many improvements, antivenoms exhibit acceptable safety profiles [1, 9, 10]. Nevertheless, antivenom quality still varies widely depending on the producer, while some antivenoms exhibit adverse reaction rates of less than 10%, others have values of greater than 50% [11, 12].”
https://jvat.biomedcentral.com/articles/10.1186/s40409-018-0144-0
In is interesting to note that there are many factors that are said to influence the severity of a venomous snakebite including the age, sex, and health of the person bitten as well as the type of snake, the geographical location of the snake, the season the bite occurred in, what the snake ate, and how recently the snake released its venom. Antivenoms themselves have been shown to have varying effects in quality due to the geographical location of the snake which somehow renders the antivenom ineffective and even dangerous in different countries and continents, even against the same type of snake. It is said that this has kept locals from seeking out medical care and sticking to traditional healers:
“Snake venoms are highly complicated. At least 26 separate enzymes have been identified with 10 of these enzymes common to all snake venoms (though in different concentrations). All snake bites are not equal. The quality of venom depends not only on the type of snake but on the season, the geographical region, the age of the snake, and how recently it has released venom previously.”
https://www.vin.com/apputil/content/
Antivenom’s fatal flaw
“A study led by Dr Fry has found that antivenoms produced using snakes from one region may perform poorly or fail completely against the same species of snakes from other regions.
Researchers tested the effectiveness of two African and two Indian saw-scaled viper antivenoms against saw-scaled vipers from 10 regions.
The results showed that the two African antivenoms were only effective against snakes from restricted ranges.
One antivenom performed well against West African saw-scaled vipers and the other performed best against the East African saw-scaled vipers.
The Indian antivenom only worked against saw-scaled vipers from the region where the antidote was produced and failed against toxins from other Indian regions. It failed completely against African saw-scaled vipers.
“These antivenoms are being sold and used interchangeably to treat all saw-scaled viper bites, and in many cases they are not working,” Dr Fry says.
“In Kenya, snakebite deaths have increased dramatically after hospitals switched supplies of a very effective African antivenom with a cheaper Indian variety.”
“This creates a knock-on effect in these communities. It’s hard enough to convince people living in these regions not to go to traditional healers to treat snakebite. And if someone does seek proper medical care but dies because of ineffective antivenom, it will be even harder to convince the next victim to seek out antivenom.”
Viper venom’s lethal evolution
CONTINUED...






 Sat Mar 23, 2024 11:33 pm by globalturbo
Sat Mar 23, 2024 11:33 pm by globalturbo Bill Haast – repeated snake bite victim from the world’s deadliest snakes tragically died at the young age of 100 from natural causes.
Bill Haast – repeated snake bite victim from the world’s deadliest snakes tragically died at the young age of 100 from natural causes. 

