FDA Rejects Neuralink Clinical Study Application and Then Immediately Grants Clearance for Human Use

KAREN KINGSTON
June 6, 2023: According to a March 2, 2023 REUTERS article, the FDA rejected Neuralink’s Premarket Approval (PMA) application to test brain chips in humans.
Per the article, Kip Ludwig, former NIH program director for neural engineering stated, “Neuralink doesn’t appear to have the mindset and experience that’s needed to get this to market anytime soon.”
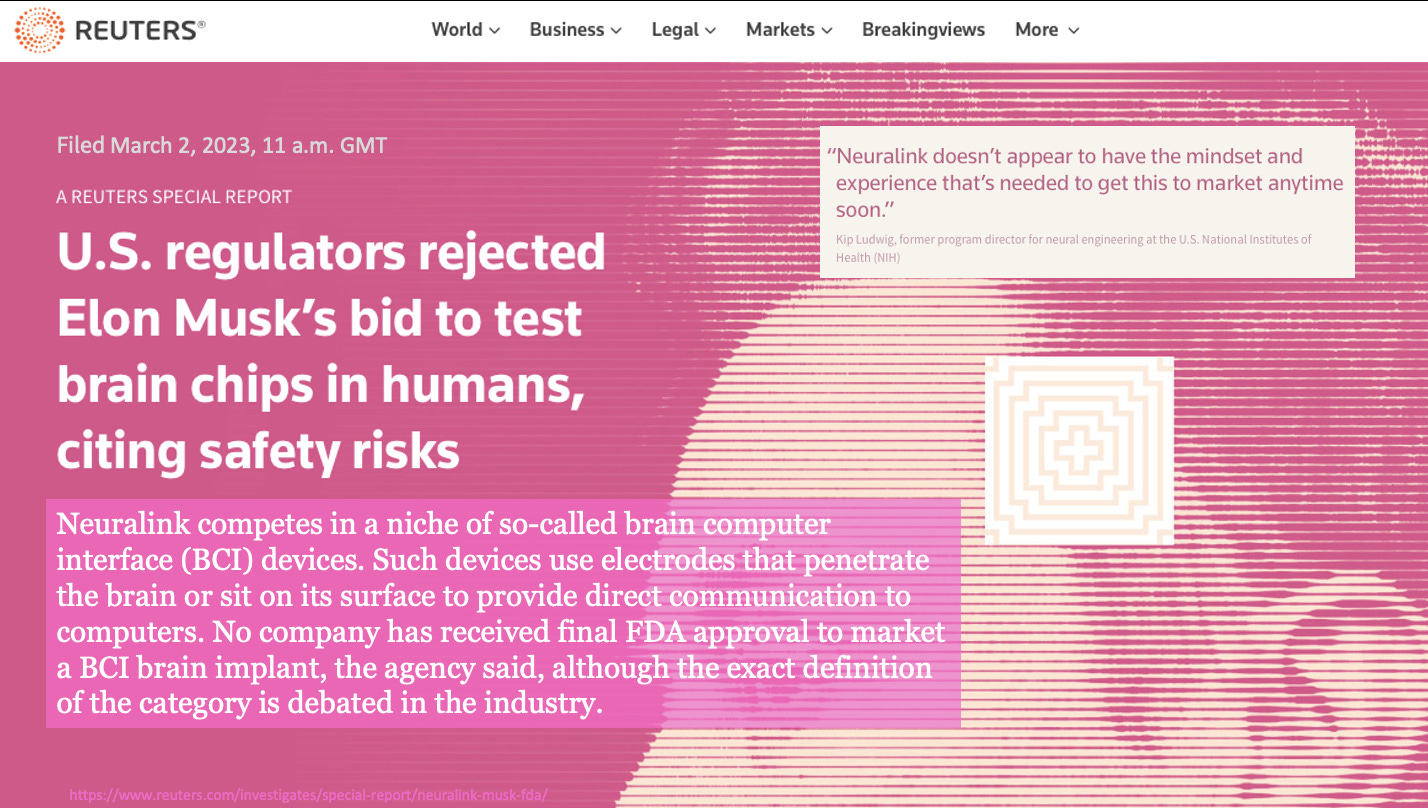
Neuralink belongs to the category of brain computer interface (BCI) devices that use electrodes to penetrate and rewire the brain. Per the article, no company has ever received final FDA approval (PMA approval) to market a BCI implant such as Neuralink.
The FDA outlined dozens of issues Neuralink needed to address before human testing, a critical milestone to receive FDA PMA approval. The FDA’s rejection of Neuralink’s PMA application was reported by REUTERS on March 2, 2023.
How did Neuralink receive FDA clearance for human use less than 30 days later?
THANKS TO: https://newhumannewearthcommunities.wordpress.com/2023/06/05/fda-rejects-neuralink-clinical-study-application-and-then-immediately-grants-clearance-for-human-use/
The FDA recently rejected Neuralink’s PMA application for human clinical trials citing dozens of safety issues. How did Neuralink receive FDA clearance for human use less than 30 days later?

KAREN KINGSTON
June 6, 2023: According to a March 2, 2023 REUTERS article, the FDA rejected Neuralink’s Premarket Approval (PMA) application to test brain chips in humans.
Per the article, Kip Ludwig, former NIH program director for neural engineering stated, “Neuralink doesn’t appear to have the mindset and experience that’s needed to get this to market anytime soon.”

Neuralink belongs to the category of brain computer interface (BCI) devices that use electrodes to penetrate and rewire the brain. Per the article, no company has ever received final FDA approval (PMA approval) to market a BCI implant such as Neuralink.
The FDA outlined dozens of issues Neuralink needed to address before human testing, a critical milestone to receive FDA PMA approval. The FDA’s rejection of Neuralink’s PMA application was reported by REUTERS on March 2, 2023.
How did Neuralink receive FDA clearance for human use less than 30 days later?
THANKS TO: https://newhumannewearthcommunities.wordpress.com/2023/06/05/fda-rejects-neuralink-clinical-study-application-and-then-immediately-grants-clearance-for-human-use/






 Sat Mar 23, 2024 11:33 pm by globalturbo
Sat Mar 23, 2024 11:33 pm by globalturbo

